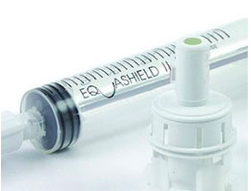
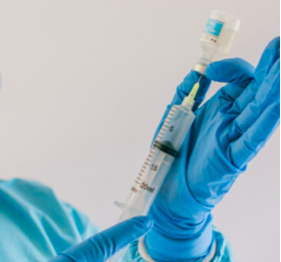
We are the exclusive distributor of EQUASHIELD II®, a market leader in Closed System Transfer Devices. EQUASHIELD II® has a double membrane design and is the only self contained pressure equalization drug transfer device that provides complete isolation of the vial while maintaining the sterile integrity of the medication. Unlike other devices that use standard syringes with open cylinders and plungers, EQUASHIELD’s distinctive double jacket syringe enclosure makes it the only CSTD to address this issue and eliminate the possibility of exposure through the back of the syringe. EQUASHIELD’s fixed fully shielded needles provide the ultimate protection from accidental needle stick injuries.
Third Party NAPRA Certified Training
IOS provides third party certifications services on your site to maximize certification techniques with your actual workflow. We offer a Master Class Two- Day Certification Course in Aseptic Training Techniques based on NAPRA Standards. The 2 days master class participants will receive didactic and live aseptic technique training and coaching based on the preparation of low and medium risk sterile compounded products, followed by testing which includes media fill for low and medium risk level CSPs, gloved finger tip sampling, and surfaces sampling (there is also high risk level media fill testing). We also offer a Recertification Course. This is offered to people who have at some time completed the Master Class. In this one day session, participants will also be presented with an emerging issue in pharmacy compounding, followed by a refresher of aseptic technique, media fill testing, gloves finger tip testing, and surface sampling.
Third Party NAPRA Certified Training Brochure
CompoundingQMS™
 IOS offers the most comprehensive NAPRA compliant electronic Quality Management System in the market. CompoundingQMS™ is the first and most customizable cloud-based solution incorporating best practices that address multiple quality, safety and compliance challenges of pharmacies. Our Quality Management System is the result of years of experience in pharmacy audit and quality control and was built from the ground up as a totally electronic cloud-based audit and quality assurance system. CompoundingQMS™ replaces inefficient paper and spreadsheet compliance programs and less customizable web-based tools. The platform addresses all NAPRA guidelines. The system has the ability to create tasks and/or alerts and real-time alerts, assign and prioritize tasks, assign follow up tasks, trace electronic signature and create reports & statistics.
IOS offers the most comprehensive NAPRA compliant electronic Quality Management System in the market. CompoundingQMS™ is the first and most customizable cloud-based solution incorporating best practices that address multiple quality, safety and compliance challenges of pharmacies. Our Quality Management System is the result of years of experience in pharmacy audit and quality control and was built from the ground up as a totally electronic cloud-based audit and quality assurance system. CompoundingQMS™ replaces inefficient paper and spreadsheet compliance programs and less customizable web-based tools. The platform addresses all NAPRA guidelines. The system has the ability to create tasks and/or alerts and real-time alerts, assign and prioritize tasks, assign follow up tasks, trace electronic signature and create reports & statistics.
Qleanspace™
The compounding of sterile preparations requires the highest quality standards in order to ensure preparation quality and safety. Parenteral therapies are becoming more complex, and patients may now receive continuous antibiotic therapy or chemotherapy, among other therapies, for several days at home. Consequently, greater attention must be paid to the environment in which these preparations are prepared, the training of personnel and quality assurance procedures to prevent complications and to protect the public more generally. QleanSpace™ meets all NAPRA standards.
QleanSpace™ provides innovative panel and nonporous materials that increase cleanliness and visibility into work areas and offers continuous monitoring of airborne particles, air pressure, temperature and humidity along with data recording and alarms. Best of all, QleanSpace™ is NAPRA compliant.
Pactosafe
Today’s effective cytostatics for cancer treatment saves lives but involve risks for the staff who have to handle waste products that are hazardous to health. The same applies to the handling of antibiotics and anti-viral medications. A number of studies show that even drug vials are contaminated on delivery. This also applies to drugs handled in closed systems. At pre-schools and retirement homes for the elderly, the sheer volume of diapers and incontinence pads constitutes a working environment issue. Handling waste in a closed system is therefore essential in order to prevent the spread of odours and dangerous aerosols. The waste sealing unit Pactosafe guarantees high levels of safety by securely sealing the waste in airtight bags. The system more than satisfies the national and international guidelines for handling waste throughout the entire waste chain.
FIELDS OF APPLICATION
- Pharmacies – preparation of cytostatic, anti-viral and antibiotics.
- Oncology wards – treatment with CMR drugs.
- Dialysis clinics and blood donor centres.
- Infectious diseases clinics.
- Retirement homes and pre-schools – handling of incontinence pads and diapers.
- Laboratories – microbiology, bacteriology, genetic engineering, pathology.